- Revenue reached RMB 2,124 million, an increase of 114% compared with Year 2022
- Adjusted net profit reached RMB 412 million, an increase of 112% compared with Year 2022
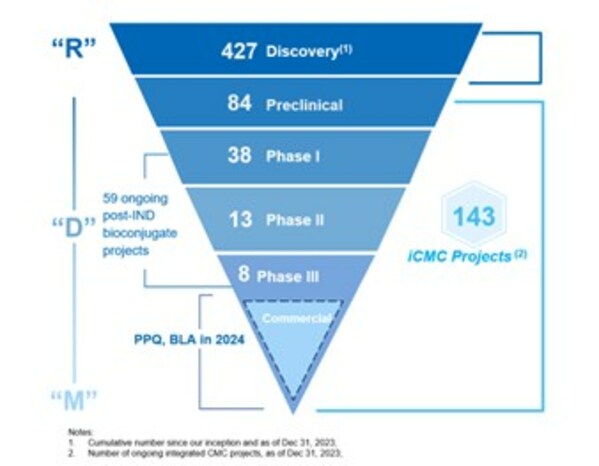
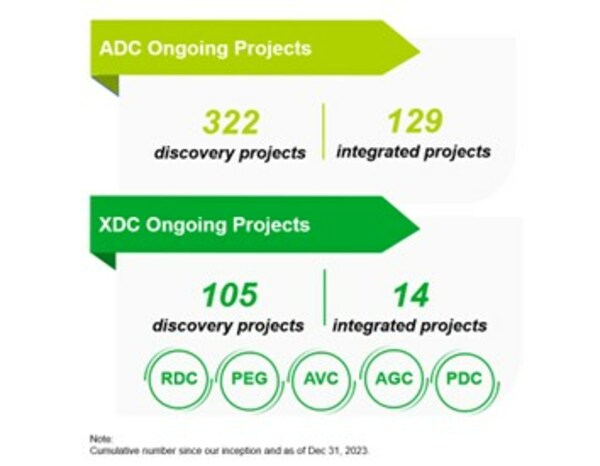
- Total number of integrated projects increased to 143
- 50 new integrated projects signed in 2023
- Number of Phase II and Phase III projects reached 21, including 5 PPQ projects
- Backlog grew to US$579 million, increase by 82%
- “All-in-One” manufacturing facilities operational in Wuxi city site with expanded capacities
- Successful completion of Hong Kong IPO, raising US$520 million, and named “Best IPO” by IFR Asian Award 2023
- Won “2023 Best CDMO” Award of World ADC Awards
- A live conference call will be hosted on March 26, 2024, at 8:00 AM Hong Kong time
SHANGHAI, March 25, 2024 /PRNewswire/ — WuXi XDC Cayman Inc. (“WuXi XDC” or “the Group”, stock code: 2268.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO) focused on ADC and the other types of bioconjugate market, is pleased to announce its annual results and corporate updates for the year ended 31 December 2023.
Dr. Jimmy Li, CEO of WuXi XDC, remarked, “The year 2023 marked a significant milestone for WuXi XDC. With the successful implementation of “Enable, Follow and Win the Molecule” strategy, we achieved outstanding performance with strong 114% YoY growth in revenue. Our unwavering commitment to excellence and innovation in the ADC and broader bioconjugation CRDMO sector underpinned our fruitful accomplishments across various areas. Notably, the Company was successfully listed on the Hong Kong Stock Exchange, signaling a new chapter in our journey. In the fast-growing ADC and broader bioconjugate industry, we continued to innovate our technology platform, and built all-in-one manufacturing facilities at our Wuxi city site, positioning us at the forefront of industry excellence. We take immerse pride in being recognized by the World ADC Awards, a testament to our team’s dedication and the trust our customers place in us. Our meticulously executed “global dual sourcing strategy” to build a new manufacturing facility in Singapore has seen a ground-breaking milestone. As we continue to enhance our capabilities and expand our capacity, we are confident in solidifying our growth in the future. Looking ahead, we remain committed to pioneering innovations, expanding our global footprint, and advancing our mission to serve patients worldwide.”
2023 Financial Highlights
Revenue
The Group’s revenue increased to RMB 2,124 million with an increase of 114% YoY. The increase was mainly attributable to (i) the growth in the number of customers and projects, driven by rapid growth of the global ADC and broader bioconjugate outsourcing service market and the Group’s established position as a leading ADC CRDMO service provider in that market and (ii) the advancement of many Group’s projects into later stage and external projects into pipeline due to effective implementation of “win the molecule” strategy.
Gross profit and gross profit margin
The Group’s gross profit increased by 114% YoY to RMB 560 million, with a gross profit margin of 26.3%, primarily driven by the strong revenue growth.
Adjusted net profit
Adjusted net profit for the period increased by 112% YoY to RMB 412 million. Margin of adjusted net profit remained stable at 19.4%.
Adjusted Diluted earnings per share (EPS)
Adjusted diluted EPS was RMB 0.38, up by 73% YoY.
2023 Business Highlights
- Our rapidly expanding and high-quality customer base has reached a cumulative total of 345, including global pharma and innovative biotech companies. Collectively, our clients have submitted 55 INDs with the strong support from our Group. Notably, 6 out of top 10 global pharmaceutical companies have actively partnered with us to develop ADC or XDC assets.
- The “Enable, Follow and Win the Molecule” strategy continued to drive sustainable and fast project growth. We signed 50 new integrated projects in 2023. As of December 31 2023, the Group’s total ongoing integrated projects reached 143.
- Later-stage projects (Phase II and III) increased to 21 with 5 PPQ projects, setting the stage for the next chapter of growth.
- The Group has accomplished a diversified project base, covering both innovative ADC and broader bioconjugate projects. Total number of integrated ADC projects reached to 129. Number of integrated XDC projects increased to 14.
Technology Expertise and External Partnerships
- Evolved from WuXiDAR4™ technology platform, the Group has developed and introduced an upgraded proprietary version, WuXiDARx™ technology, aiming to meet clients’ demands to develop highly homogeneous ADCs with a range of distinct DAR values. The products produced from the platform have enhanced stabilities and much better pharmacokinetic profiles.
- The Group announced multiple partnerships with prominent biotech or pharmaceutical companies, e.g., the partnership with GeneQuantum, SyntaBio, Multitude Therapeutics/HySlink and Intocell, to accelerate the discovery of preclinical ADC candidates (PCCs) and develop more novel bioconjugates, and improve the development efficiency and rate of success.
Capacity Expansion & Quality System
- The Group currently operates three sites in Wuxi, Changzhou and Shanghai. The Wuxi city site has reached an important milestone by realizing fully integrated “All-in-One” manufacturing within one single site. The site now can manufacture all four ADC components, i.e., mAb intermediate, payload-linker, bioconjugate drug substance (DS) and bioconjugate drug product (DP). This will greatly simplify the ADC supply chain and significantly shorten the manufacturing cycle time.
- The global uniquely designed dual-function line for mAb intermediate and bioconjugate drug substance is efficiently operational. It accelerates processes, reduces costs, streamlines workflows, and shortens project timelines.
- All company’s manufacturing operations are compliant with GMP regulation of FDA, EMA, and NMPA, with 100% success on client and QP audits in 2023, which will ensure the innovative bioconjugate products manufactured in high quality.
- Construction of Singapore site for both clinical and commercial manufacturing is on track with ground-breaking in March 2024 and operation expected to commence in 2026.
Industry and Capital Market Recognition, WBS Lean Management and ESG Dedication
- The Group has been named the winner of the “2023 Best Contract Development Manufacturing Organization (CDMO)” Award of World ADC Awards, demonstrating continual enhancement of industry-leading technology platform, outstanding capabilities and the trust our customers place in us.
- “Best IPO” of the year 2023 by IFR Asia Awards and being the largest Healthcare HK IPO since 2021, raising over US$520million in total and supported by high quality cornerstone investors.
- Guided by the Group’s lean culture and enabled by the WBS (WuXi Business System), the Group integrated lean management principles and methods to drive continuous improvement and dedicated to improving the comprehensive competitiveness from multiple dimensions including quality, process, cost and efficiency.
- The Group continues to improve ESG governance and has established an ESG Committee chaired by the CEO. The Group has been actively creating long-term value for the industry and society, focused on talent development, women’s empowerment and employee health, and achieved a number of ESG goals by considering ESG as a cornerstone of sustainable business development.
Key Financial Ratios
(For the Twelve Months Ended Dec 31)
|
Key Financial Ratio |
2023 |
2022 |
YoY% |
|
Revenue (In RMB million) |
2,124 |
990 |
114 % |
|
Gross Profit (In RMB million) |
560 |
261 |
114 % |
|
Adjusted EBITDA (In RMB million) Margin (%) |
501 23.6% |
264 26.6% |
90 % |
|
Adjusted Net Profit (In RMB million) Margin (%) |
412 19.4% |
194 19.6% |
112 % |
|
Adjusted Diluted EPS (In RMB) |
0.38 |
0.22 |
73 % |
About WuXi XDC
WuXi XDC Cayman Inc. (“WuXi XDC”, stock code: 2268.HK) is a leading global CRDMO focused on antibody drug conjugates (ADC) and the broader bioconjugate market. It provides end-to-end contract research, development and manufacturing services for bioconjugates, including ADCs. Its services cover antibody intermediates and other biologics intermediates, chemical payloads and linkers, as well as bioconjugate drug substances and drug products. For more information about WuXi XDC, please visit: wuxixdc.com
Contacts
Investor: wuxixdc.ir@wuxibiologics.com
Media: wuxixdc_pr@wuxibiologics.com
BD: wuxixdc_info@wuxibiologics.com
Use of Adjusted Financial Measures (Non-IFRS Measures)
We have provided adjusted net profit, adjusted net profit margin, adjusted EBITDA, adjusted EBITDA margin and adjusted diluted earnings per share for the corresponding periods, which excludes the share-based compensation expenses, listing expenses, gains or losses from equity investments and foreign exchange gains or losses, and are not required by, or presented in accordance with, IFRS. We believe that the adjusted financial measures used in this presentation are useful for understanding and assessing underlying business performance and operating trends, and we believe that management and investors may benefit from referring to these adjusted financial measures in assessing our financial performance by eliminating the impact of certain unusual and non-recurring items that we do not consider indicative of the performance of our business. However, the presentation of these non-IFRS financial measures is not intended to be considered in isolation or as a substitute for the financial information prepared and presented in accordance with IFRS. You should not view adjusted results on a stand-alone basis or as a substitute for results under IFRS, or as being comparable to results reported or forecasted by other companies.